Table of Contents
- What Makes Graphene Production Unique
- Main Methods Used to Make Graphene
2.1 Mechanical Exfoliation
2.2 Chemical Vapour Deposition
2.3 Liquid Phase Exfoliation
2.4 Reduction of Graphene Oxide
2.5 Plasma Enhanced Methods
2.6 Electrochemical Exfoliation - How Purity and Quality Affect Graphene Performance
- Industrial-Scale Graphene Production: Current State and Near-Term Outlook
4.1 Why The Production Method Matters
Graphene has earned a reputation as the wonder material of the modern world. It is stronger than steel, lighter than a whisper, and conducts electricity with a kind of smooth confidence that would impress any engineer. With so many bold claims flying around, it is natural to ask the question that sits underneath them all. How do we actually make this stuff?
It turns out that graphene has more than one path into existence. Some paths are gentle and elegant, like peeling a page from a book. Others involve giant heated chambers, intricate chemistry, and enough laboratory equipment to make your inner scientist grin. This guide walks you through the major ways graphene is made today, from small-scale experiments to industrial production.
If the universe ever wanted to give us a lesson in how something so thin could reshape the future, graphene would be the perfect example.
1. What Makes Graphene Production Unique
Graphene is a single layer of carbon atoms arranged in a hexagonal pattern. That is it. It is the simplest thing imaginable, yet producing it in a clean, consistent way can be surprisingly tricky.
Every production method tries to do one of two things:
- Separate graphene layers from graphite.
- Build the layer from atoms in a controlled environment.
These two broad approaches create very different types of graphene. Some methods produce perfect, pristine sheets that work well in electronics. Others produce many smaller flakes that shine in batteries, coatings, and composite materials.
The important thing is that no single method dominates everything. Each one serves a different purpose and unlocks different applications.
2. Main Methods Used to Make Graphene
2.1 Mechanical Exfoliation
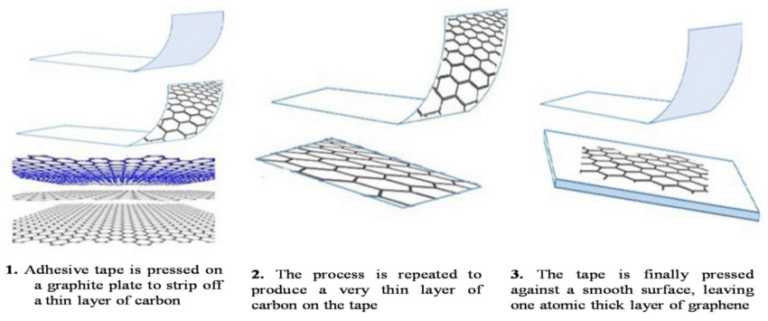
Image Source: MDPI
This method is the original storybook version of graphene production. Scientists sometimes call it the Scotch tape method because the first samples were famously isolated using adhesive tape. It sounds almost too simple, but simplicity can be powerful.
Here is the basic process:
- Take high-quality graphite.
- Press adhesive tape onto the surface.
- Pull the tape away to lift thin layers.
- Repeat this several times until only a few layers remain.
- Transfer the layers onto a substrate for study.
This method produces excellent graphene. The sheets are large, clean, and nearly perfect in structure. The problem is that you cannot exactly scale it up for factories unless you want an army of engineers peeling tape until they lose their minds.
Mechanical exfoliation helped researchers study graphene, but it stays mostly within laboratories. It is the closest thing chemistry has to a magic trick.
2.2 Chemical vapour Deposition
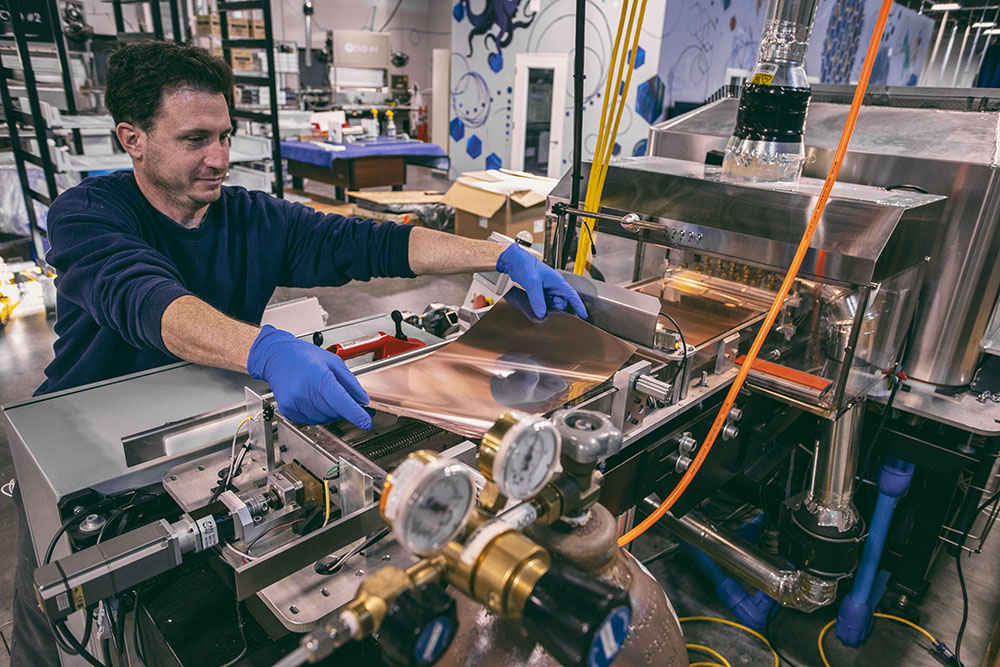
If mechanical exfoliation is art, chemical vapour deposition is engineering. CVD is the leading method for producing large sheets of high quality graphene suitable for electronics, sensors, and advanced materials.
The process happens inside a heated chamber where methane gas and other carbon containing molecules flow over a metal surface such as copper or nickel. At high temperatures, the gas breaks apart and carbon atoms settle onto the metal in a precise, honeycomb pattern.
The CVD method typically works like this:
- Prepare the metal substrate by cleaning it thoroughly.
- Heat the substrate in a controlled furnace.
- Flow in a carbon based gas that breaks apart at high temperature.
- Allow the carbon atoms to arrange themselves into a graphene sheet.
- Cool the system and remove the film.

Image Source: www.nature.com
If this sounds like cooking, you are not entirely wrong. The trick is keeping the temperature stable, the gas composition consistent, and the environment clean. When everything works smoothly, CVD produces beautiful, continuous films.
The final step is usually to transfer the graphene onto another surface, which requires delicate handling. Scientists spend a surprising amount of time removing wrinkles from these layers. It is a little like doing laundry at the atomic scale.
2.3 Liquid Phase Exfoliation
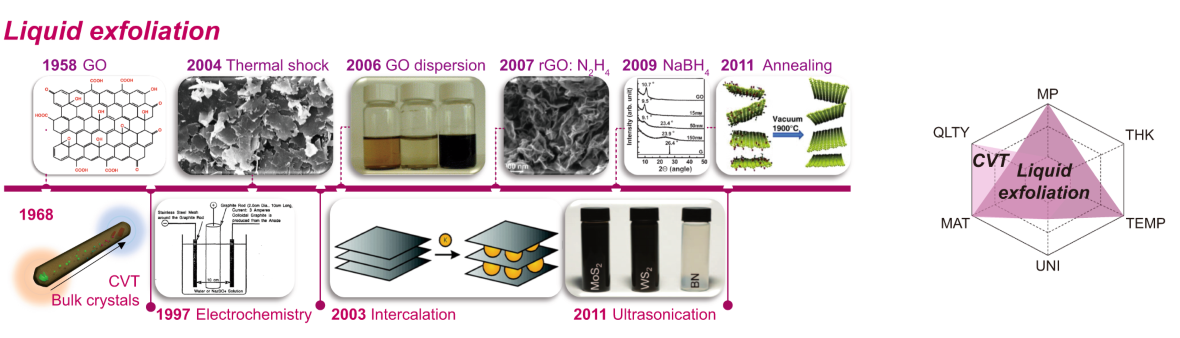
Image Source: www.nature.com
Liquid phase exfoliation takes a more practical approach. Instead of tape or heated chambers, it uses liquids and mechanical energy to separate graphene layers from graphite powder.
Producers might suspend graphite in a solvent, apply ultrasonic vibrations, and allow the layers to peel away from each other. When done correctly, this yields millions of small graphene flakes floating in a liquid dispersion.
This method is popular because it is:
- Scalable.
- Cost effective.
- Compatible with inks and coatings.
The resulting graphene is not perfectly structured, but that is not a problem for many applications. Printed electronics, conductive coatings, composite materials, and sensors all work well with exfoliated graphene flakes.
If CVD is the fine dining of graphene production, liquid phase exfoliation is the hearty home-cooked meal. It gets the job done, and it does so in bulk.
2.4 Reduction of Graphene Oxide
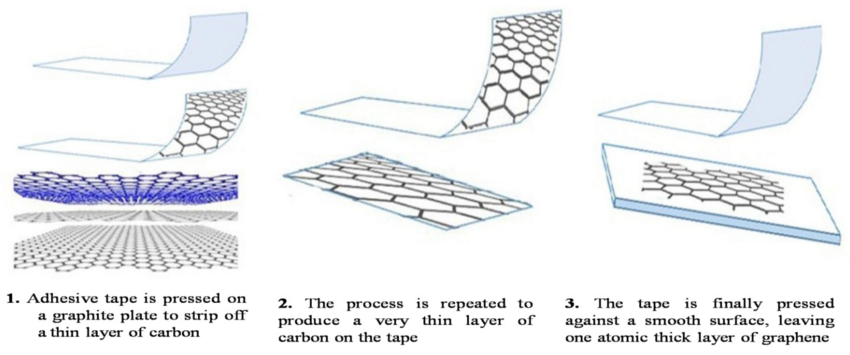
Image Source: www.layeronematerials.com
Another route begins with graphite oxidized into graphene oxide. Graphene oxide can be dispersed in water like a friendly guest who never causes trouble. After creating this dispersion, manufacturers apply heat or chemicals to remove some of the oxygen groups. This produces reduced graphene oxide.
This type of graphene contains small structural defects. That might sound like a flaw, but it actually makes reduced graphene oxide incredibly useful for:
- Batteries and supercapacitors.
- Energy storage materials.
- Conductive films.
- Polymer composites.
This method is scalable and widely used. While reduced graphene oxide is not the purest form of graphene, it fills a huge part of the commercial market.
2.5 Plasma Enhanced Methods
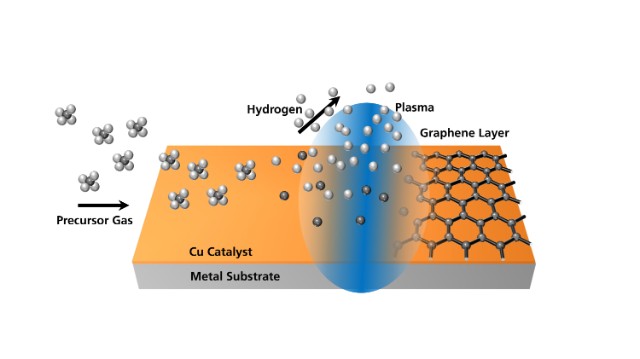
Image Source: www.thegraphenecouncil.org
Plasma enhanced chemical vapour deposition combines electric fields with gas based graphene growth. The plasma helps carbon atoms settle onto the substrate at lower temperatures, which allows researchers to use more types of surfaces.
The main advantages include:
- Lower energy requirements.
- Greater substrate flexibility.
- Faster growth times.
Plasma enhanced methods are still advancing, but they offer a promising route for future electronics, sensor platforms, and more flexible materials.
2.6 Electrochemical Exfoliation
Electrochemical exfoliation uses electricity to separate graphene layers from graphite. A piece of graphite is placed in an electrolyte solution, and a voltage is applied. The ions begin to move between the layers and push them apart.
This approach produces graphene flakes with good conductivity and fewer defects than many chemical routes. It also produces graphene more quickly and with fewer harsh chemicals, which makes it appealing for sustainable manufacturing.
Although it is still developing, this technique is finding its way into energy storage and mass production workflows.

3. How Purity and Quality Affect Graphene Performance
Not all graphene looks the same under a microscope. Some forms are close to perfect. Others are slightly fractured or oxidized. The quality affects conductivity, mechanical strength, and chemical behavior.
Producers usually consider:
- Number of layers.
- Sheet size.
- Structural defects.
- Functional groups attached.
- Level of oxidation.
High quality graphene works in electronics and optical devices. Lower cost versions work in batteries, coatings, and composites. The best method depends entirely on the intended application.
This diversity is what makes graphene such a flexible technology. It can be tailored for industries that need perfection and industries that need practicality.
4. Industrial-Scale Graphene Production: Current State and Near-Term Outlook
By 2026, graphene production has become far more mature than it was even a decade ago. Factories now produce:
- Square meter CVD films for sensors and coatings.
- Kilograms of exfoliated graphene for inks and composites.
- Tons of graphene oxide for batteries, membranes, and water treatment.
Companies have optimized solvent recycling, chemical waste reduction, and continuous flow oxidation to reduce cost. Automated systems now control temperature, flow rate, and reaction timing with a level of precision early graphene researchers could only dream of.
The result is a growing market that blends practicality with innovation.
4.1 Why The Production Method Matters
Each production approach shapes the future of graphene. When companies choose how to make graphene, they are really choosing the kinds of products that material can serve.
CVD graphene pushes innovation in flexible electronics and optical devices. Exfoliated graphene builds stronger composites and lightweight coatings.
Graphene oxide and reduced graphene oxide power new energy storage devices. This is why understanding production is so important. It tells you where the breakthroughs will appear and which industries will benefit first.
Curious about how graphene is solving six major engineering challenges, the differences between graphene and graphene oxide, or breakthroughs in bone repair? Check out some of our other posts to learn more.