Basics-Discovery
Atomic Structure and Basic Properties
Graphene is an extremely thin carbon material consisting of a single layer of tightly-bonded carbon atoms arranged in a hexagonal pattern resembling chicken wire, or a honeycomb.. Each atom connects with three others, giving this two-dimensional material remarkable strength and lightness.
Due to this unique honeycomb-lattice structure, graphene is highly effective at conducting both electricity and heat, outperforming most traditional materials. These exceptional properties make graphene essential for advanced nanotechnology and continued future developments in the material sciences.
The Discovery of Graphene & the Nobel Prize
The groundbreaking discovery of graphene took place in 2004 at the University of Manchester. Andre Geim and Konstantin Novoselov successfully isolated this two-dimensional material, using a surprisingly simple mechanical exfoliation technique using commonly found adhesive tape.
Their innovative work demonstrated the stable existence of monolayer graphene and revealed its exceptional electrical and thermal properties.
This fundamental research had a profound impact on materials science and nanotechnology, leading to their subsequent recognition by being awarded the Nobel Prize in Physics in 2010 for their pioneering work.
Variants & Comparisons
Comparison with Other Carbon Allotropes
Graphene, a two-dimensional carbon allotrope, stands apart from other forms like diamond, graphite, and fullerenes due to its unique structure and properties. Standing apart from the rigid, three-dimensional network of diamond or the layered structure of graphite, graphene is one, single atomic sheet.
This arrangement provides graphene with exceptional electrical and thermal conductivity, superior to that of graphite’s delocalized electrons, and remarkable mechanical strength.
While fullerenes (like buckyballs and carbon nanotubes) are also nanoscale carbon structures, their curved, closed-in forms make them altogether different from graphene’s sheet structure, impacting their direct applications in nanotechnology.
Variants: Monolayer, Few‑layer, Nanotubes, & Graphene Oxide
If graphene is one thing, it is versatile: it extends to various forms, each with distinct characteristics for diverse nanotechnology applications.
- Monolayer graphene is a single atomic sheet, exhibiting the purest form of its exceptional electrical conductivity and mechanical strength.
- Few-layer graphene (FLG) consists of several graphene sheets stacked together, offering tunable properties that bridge the gap between monolayer and bulk graphite.
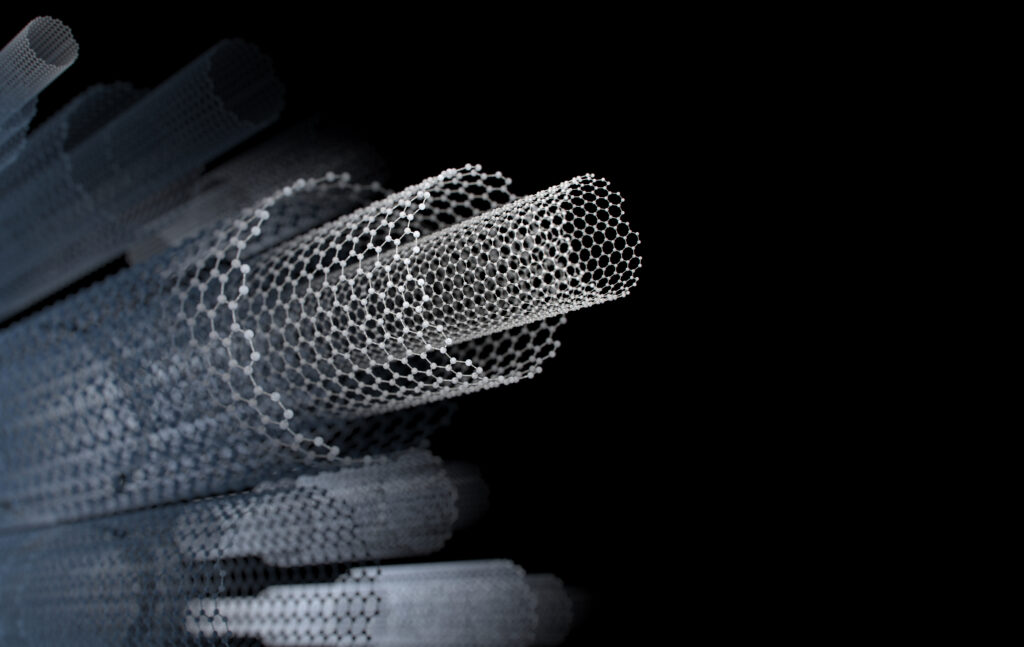
- Carbon nanotubes (CNTs) are essentially graphene sheets rolled into seamless cylinders, renowned for their incredible strength and electrical transport.
- Graphene oxide (GO) is a chemically modified graphene derivative with oxygen-containing groups, making it dispersible in water and easily processed for applications like sensors and composites, often serving as a precursor for restoring graphene’s conductivity.
Properties & Characterization
Unique Physical & Chemical traits
Graphene’s extraordinary physical and chemical properties, due to its unique atomic structure, allow electrons to flow across the lattice unimpeded by other layers, moving with extraordinary speed and freedom. Graphene can carry a thousand times more electricity than copper, making it a promising material for advanced electronics.
Furthermore, graphene exhibits remarkable tensile strength, being significantly stronger than steel, despite its minimal thickness, due to its single carbon sheet.
This robustness is coupled with impressive flexibility, allowing it to be bent and stretched without any damage.
The combined traits of superior conductivity, immense strength, and inherent flexibility make graphene highly sought after for diverse nanotechnology applications and next-generation materials.
Material Characterization Techniques
If graphene is one thing, it is versatile: it extends to various forms, each with distinct characteristics for diverse nanotechnology applications.
- Monolayer graphene is a single atomic sheet, exhibiting the purest form of its exceptional electrical conductivity and mechanical strength.
- Few-layer graphene (FLG) consists of several graphene sheets stacked together, offering tunable properties that bridge the gap between monolayer and bulk graphite.
- Carbon nanotubes (CNTs) are essentially graphene sheets rolled into seamless cylinders, renowned for their incredible strength and electrical transport.
- Graphene oxide (GO) is a chemically modified graphene derivative with oxygen-containing groups, making it dispersible in water and easily processed for applications like sensors and composites, often serving as a precursor for restoring graphene’s conductivity.